
FDA 21 CFR Part 820 eLearning
600033-0201-905
- tipo de producto
- eLearning
- Tipo de entrenamiento
- eLearning
- Nivel
- 1
Learning Objective: Overview about guideline 21 CFR Part 820 Code of Federal Regulation of Food and Drug Administration (FDA)
Focus:
- purpose, scope, application area and general contents
- identification of specific requirement as per 21 CFR Part 820
- application of concepts for quality assurance
Duration: approx. 4 hours
Product note
Contents of this eLearning only available in English language.
Vistos recientemente
170,20 USDmás el IVA
Envío en > 30 días hábiles
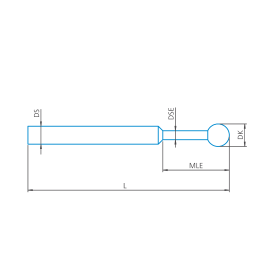
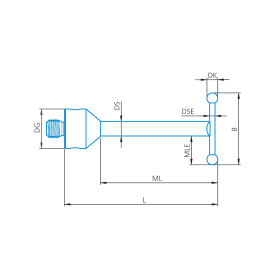
M3, Palpador recto, esfera de rubí, eje de carburo de tungsteno
626113-0200-038
49,70 USDmás el IVA
Envío en > 30 días hábiles
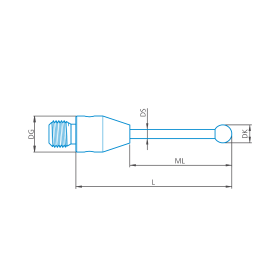
M3 XXT, Palpador escalonado, esfera de nitruro de silicio, eje de carburo de tungsteno
626113-0102-060
60,30 USDmás el IVA
Envío en > 30 días hábiles
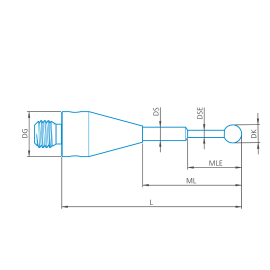
Placa adaptadora RDS para el cabezal de sonda VAST XXT
620670-9550-000
2.694,50 USDmás el IVA
Envío en > 30 días hábiles
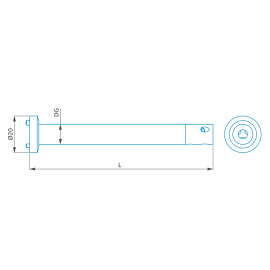
Extensión con receptor de cono, ThermoFit Pro
626107-4245-000
322,40 USDmás el IVA
Envío en > 30 días hábiles
243,60 USDmás el IVA
Envío en > 30 días hábiles
CMG PRISMO, 700 X 1000 mm, 1 placa, M6, rejilla de 50 mm
600062-0210-300
2.331,90 USDmás el IVA
Envío en > 30 días hábiles